Introduction
Pentane, a simple hydrocarbon with the molecular formula C₅H₁₂, is an excellent example to introduce the concept of structural isomerism in organic chemistry. Structural isomers are compounds that have the same molecular formula but different arrangements of atoms, resulting in different structures and properties. In this article, we’ll explore how many structural isomers of pentane exist, discuss what makes each one unique, and explain the reasoning behind their different structures in a clear and straightforward way.
Understanding Structural Isomers
What Are Structural Isomers?
- Definition: Structural isomers are compounds that share the same molecular formula but differ in the connectivity of their atoms.
- Why It Matters: These differences can lead to variations in physical and chemical properties even though the compounds contain the same types and numbers of atoms.
The Case of Pentane
Pentane has the formula C₅H₁₂. The key to determining its structural isomers is to consider all the possible ways the five carbon atoms can be connected without breaking the rules of valency (each carbon forming four bonds).
The Three Structural Isomers of Pentane
For pentane, there are exactly three structural isomers:
1. n-Pentane (Normal Pentane)
- Structure: A straight-chain arrangement where all five carbon atoms are connected in a single continuous chain.
- Diagram:
CH3 – CH2 – CH2 – CH2 – CH3 - Characteristics:
- It is the simplest form of pentane.
- It has the highest boiling point among the three isomers due to stronger van der Waals forces in its extended shape.
2. Isopentane (Methylbutane)
- Structure: A branched isomer where the main chain has four carbon atoms, with a methyl (CH₃) group attached to the second carbon.
- Diagram:
CH3 | CH3 – CH – CH2 – CH3 - Characteristics:
- The branching makes isopentane more compact compared to n-pentane.
- It typically has a lower boiling point due to decreased surface area for intermolecular forces.
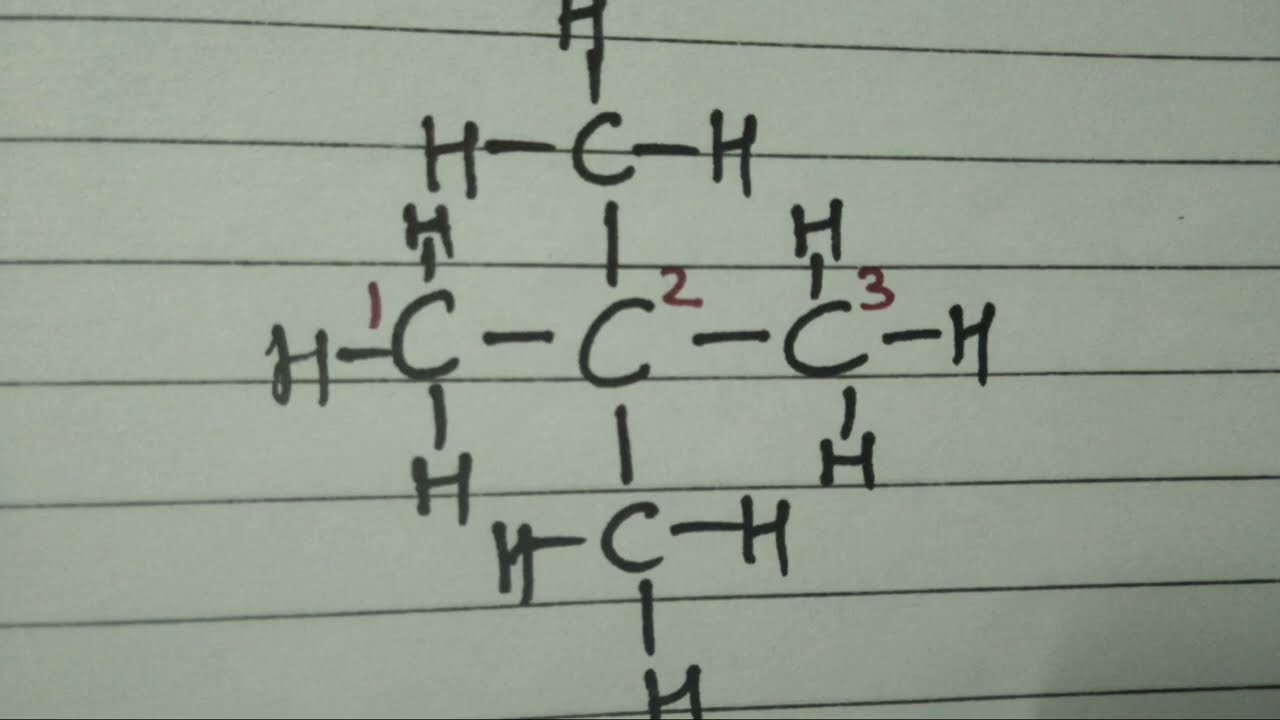
3. Neopentane (Dimethylpropane)
- Structure: A highly branched isomer where a central carbon atom is bonded to four methyl groups. It can be seen as a propyl backbone with two additional methyl groups attached to the central carbon.
- Diagram:
CH3 | CH3 – C – CH3 | CH3 - Characteristics:
- Neopentane is the most compact isomer of pentane.
- It has the lowest boiling point among the three due to its highly symmetrical and compact shape, which results in very weak intermolecular interactions.
Why Only Three Isomers?
When drawing structural isomers for pentane (C₅H₁₂), we consider the following factors:
- Carbon Connectivity:
There are only a limited number of ways to connect five carbon atoms that satisfy the tetravalency (each carbon forming four bonds) and maintain a stable structure. - Symmetry and Redundancy:
Some arrangements that might look different at first glance are actually the same molecule due to the symmetry of the structure. For pentane, these symmetries limit the number of unique isomers to three. - Exhaustive Enumeration:
Through systematic analysis or by using isomer enumeration techniques, chemists have confirmed that only three unique structures exist for pentane.
Conclusion
Pentane (C₅H₁₂) has exactly three structural isomers: n-pentane (a straight-chain structure), isopentane (methylbutane, with a single branch), and neopentane (dimethylpropane, with a compact, highly branched structure). Each isomer exhibits distinct physical properties due to differences in molecular shape and intermolecular interactions.
Understanding these isomers is a fundamental part of organic chemistry, demonstrating how the same set of atoms can create different compounds with unique characteristics. Whether you’re studying for an exam or just exploring the wonders of chemistry, the case of pentane is an excellent example of the beauty and complexity of molecular structures.
Disclaimer: This article is for educational purposes and is intended to provide a clear and accessible explanation of structural isomers in pentane. For more detailed studies, consult advanced organic chemistry textbooks or academic resources.
Also Check:
• How Many Altitudes Can a Triangle Have? An Easy-to-Understand Guide
• Can Two Adjacent Angles Be Complementary? Understanding and Illustrating the Concept
• How Many Instances Can Be Created for an Abstract Class? An In-Depth Explanation
• Can There Be Mitosis Without DNA Replication in S Phase? Exploring the Cell Cycle Fundamentals




2 thoughts on “How Many Structural Isomers Can Be Drawn for Pentane? An Easy-to-Understand Guide”